How Cells Deliver Proteins: New Cargo and Key Players Identified in Cellular Transport System
Inside the Cell’s Delivery Network: How Proteins Get to Where They’re Needed
Imagine a bustling city where every package—medicines, food, building materials—must reach the right address at the right time. Now shrink that city to the size of a single human cell. That’s the secretory pathway: a highly organized intracellular transport system that ensures proteins are correctly sorted, packaged, and delivered to their destinations.
When this system fails, the consequences can be severe—ranging from immune dysfunction to neurodevelopmental disorders.
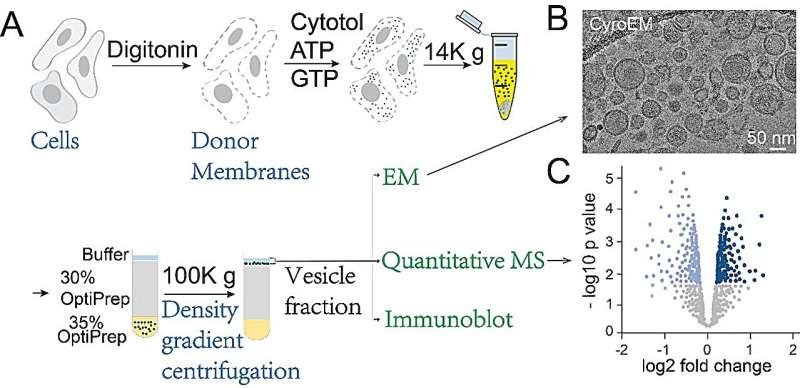
Now, researchers at the Hong Kong University of Science and Technology (HKUST) have made a major breakthrough in understanding how this cellular “postal service” works. Using an innovative combination of vesicle reconstitution, electron microscopy, and proteomics, they’ve identified new cargo proteins and critical regulatory factors involved in two key transport complexes: AP-1 and AP-4.
Their findings, published in Proceedings of the National Academy of Sciences (October 2025), offer fresh insights into rare genetic diseases—and a powerful new method to study cellular logistics.
The Trans-Golgi Network: The Cell’s Central Sorting Hub
At the heart of the secretory pathway lies the trans-Golgi network (TGN)—a dynamic organelle that acts like a distribution center. Here, newly made proteins are tagged, sorted, and loaded into tiny membrane-bound bubbles called transport vesicles.
These vesicles then ferry their cargo to:
- The cell surface (for secretion or membrane insertion)
- Lysosomes (for degradation)
- Other organelles
The accuracy of this process depends on adaptor protein (AP) complexes, molecular “barcode scanners” that recognize sorting signals on cargo proteins and help pack them into the right vesicles.
Two of these complexes—AP-1 and AP-4—are essential for TGN export. But until now, scientists didn’t know the full list of proteins they carry—or what other molecules help them do their job.
A New Toolkit to Map the Cellular Supply Chain
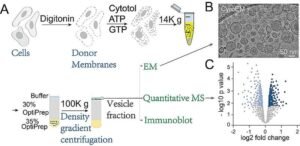
Led by Professor Guo Yusong (HKUST) and Professor Yao Zhong-Ping (PolyU), the research team developed a groundbreaking experimental platform that combines:
- In vitro vesicle reconstitution – Building functional transport vesicles in the lab
- Quantitative mass spectrometry – Identifying every protein inside those vesicles
- Electron microscopy – Visualizing vesicle structure and formation
By comparing vesicles from normal cells versus cells lacking AP-1γ1 or AP-4ε (key subunits), they could pinpoint which cargos depend on each complex.
Key Discoveries: New Cargos and Hidden Helpers
🔹 New Cargo Proteins Identified
- CAB45: A calcium-binding protein now confirmed as an AP-1-dependent cargo
- ATRAP (Angiotensin II Receptor-Associated Protein): Recognized by AP-4 via a tyrosine-based sorting signal in its tail
ATRAP helps regulate blood pressure signaling—linking vesicle trafficking to cardiovascular physiology.
🔹 Critical Accessory Factors Revealed
The team also discovered two previously unknown regulators of AP-4 function:
- WDR44: When depleted, the AP-4 cargo ATG9A (essential for autophagy) gets stuck in the Golgi
- PRRC1: Loss of this protein traps ATG9A in the endoplasmic reticulum, crippling the cell’s ability to recycle damaged components
“These proteins aren’t part of the core AP-4 complex,” explains Prof. Guo, “but they’re essential for its function—like unseen logistics coordinators.”
Why This Matters: From Basic Science to Human Disease
Mutations in AP-1 and AP-4 genes cause serious inherited disorders:
- AP-1 mutations: Linked to MEDNIK syndrome (skin, liver, and neurological defects) and X-linked intellectual disability
- AP-4 deficiency syndrome: Causes hereditary spastic paraplegia, developmental delay, and seizures
By identifying the full set of cargos these adaptors carry—like ATG9A (autophagy), ATRAP (signaling), and others—scientists can now understand why specific tissues fail in these diseases.
For example:
- If ATG9A can’t reach its destination, neurons may accumulate toxic waste → neurodegeneration
- If ATRAP is misrouted, blood pressure regulation may falter
This knowledge opens doors to targeted therapies that restore proper trafficking—even if the adaptor itself is mutated.
A Method That Changes the Game
Beyond the specific findings, the team’s integrated vesicle proteomics platform is a major advance for cell biology.
Traditional methods often rely on indirect evidence or static snapshots. This approach:
- Reconstructs functional transport events in real time
- Provides proteome-wide cargo maps
- Reveals accessory factors that genetic screens might miss
“This toolkit allows us to systematically dissect any vesicle pathway,” says Prof. Guo. “It’s not just about AP-1 and AP-4—it’s a blueprint for studying cellular transport as a whole.”
Relevance to Clinical Microbiology
While this research focuses on fundamental cell biology, it has deep implications for infectious disease and immunity:
- Many pathogens (e.g., Salmonella, Mycobacterium) hijack host vesicle trafficking to survive inside cells
- Immune cells rely on precise protein delivery to present antigens, secrete cytokines, and kill infected cells
- Defects in trafficking can mimic or exacerbate primary immunodeficiencies
Understanding the molecular logistics of the secretory pathway helps microbiologists see how microbes exploit—or disrupt—these systems.
Final Thought: Precision in the Microscopic Metropolis
Cells are not bags of soup—they’re highly organized cities with intricate supply chains. The work from HKUST reveals just how precise, and how fragile, that organization can be.
Every cargo protein delivered, every vesicle formed, depends on a network of adaptors, signals, and helpers. When one piece fails, the whole system can collapse.
But with tools like vesicle proteomics, we’re learning to map, monitor, and mend this microscopic metropolis—one protein at a time.
Stay Updated on Cellular Mechanisms & Disease
At ClinicalMicrobiology.org, we connect cutting-edge cell biology to clinical insights—because understanding how cells work is key to understanding how they fail.
👉 Subscribe today for expert coverage of trafficking disorders, host-pathogen interactions, and the molecular machinery of life.
Note: This article is based on original research from HKUST and PolyU (published in PNAS, October 2025). Scientific credit belongs to Prof. Guo Yusong, Prof. Yao Zhong-Ping, and Dr. Peng Ziqing. Content has been fully rewritten for clarity, originality, and educational value.





Post Comment