Tracking Ferroptosis in Real Time: How Glowing Antioxidants Reveal Where Cell Death Begins
Watching Cell Death Unfold: A Breakthrough in Ferroptosis Research
For years, scientists have known that ferroptosis—a recently discovered form of regulated cell death—plays a critical role in both cancer progression and neurodegenerative diseases like Alzheimer’s and Parkinson’s. But one key question remained: Where does ferroptosis actually start inside the cell?
Now, researchers at McGill University have answered that question—and in doing so, developed a revolutionary tool to watch this deadly process unfold in real time inside living cells.
Their findings, published in Nature Chemistry (October 2025), reveal that ferroptosis begins not at the cell’s outer membrane—as once suspected—but deep within two key organelles: the endoplasmic reticulum (ER) and lysosomes.
Even more importantly: protecting these structures can completely stop ferroptosis.
What Is Ferroptosis—and Why Does It Matter?
Ferroptosis is a type of iron-dependent cell death driven by the oxidative destruction of lipids (fats) in cell membranes. Unlike apoptosis (programmed cell suicide) or necrosis (traumatic cell death), ferroptosis is fueled by runaway lipid peroxidation—a chain reaction where free radicals attack unsaturated fatty acids in membranes.
This process has a dual role in human health:
- In cancer: Inducing ferroptosis can kill tumor cells that resist other forms of death.
- In neurodegeneration: Uncontrolled ferroptosis may destroy healthy brain cells, accelerating diseases like ALS, Alzheimer’s, and Parkinson’s.
But to harness or block ferroptosis therapeutically, scientists needed to know exactly where and how it begins.
The Glow That Reveals the Invisible
The McGill team, led by Dr. Gonzalo Cosa, designed custom fluorescent antioxidant probes that act like molecular flashlights.
Here’s how they work:
- The probes are “fluorogenic”—they only emit light when they neutralize harmful free radicals.
- As the antioxidants are consumed during ferroptosis, they glow brighter, revealing when and where lipid damage occurs.
- Using high-resolution live-cell imaging, researchers tracked the glow in real time.
“It’s like installing tiny sensors inside the cell that light up the moment trouble starts,” explains Cosa.
This approach provided unprecedented spatial and temporal resolution—something traditional biochemical methods couldn’t achieve.
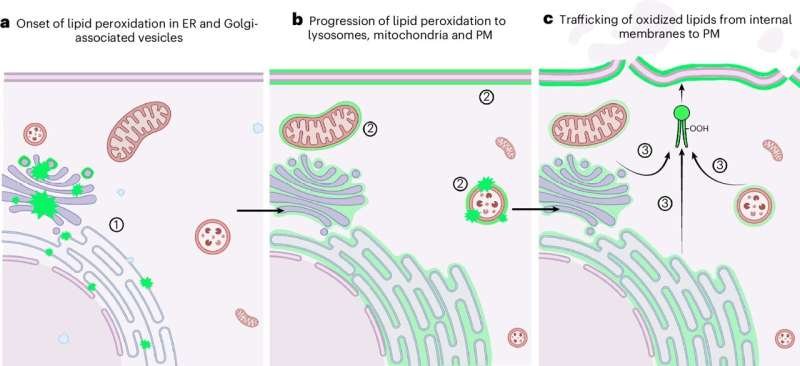
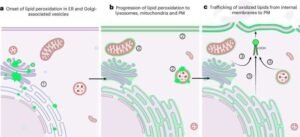
The Surprising Origin: Endoplasmic Reticulum and Lysosomes
Contrary to long-standing assumptions, the team found that lipid peroxidation begins in the endoplasmic reticulum (ER)—a network of membranes involved in protein and lipid synthesis—not in the plasma membrane.
Soon after, damage spreads to lysosomes, the cell’s recycling centers. Once both organelles are compromised, the cell rapidly collapses.
Crucially, the team tested protective strategies:
- Shielding the ER and lysosomes with targeted antioxidants halted ferroptosis completely.
- Protecting only the outer cell membrane had no effect—proving the plasma membrane is a victim, not the origin.
“This changes how we think about ferroptosis,” says Cosa. “The battle is won or lost inside the cell—not at its borders.”
Implications for Drug Development
These insights have immediate clinical relevance:
🔹 For Cancer Therapy
Drugs that induce ferroptosis (like RSL3 or erastin) are being tested in tumors resistant to conventional treatments. Now, scientists can design compounds that specifically target the ER to maximize cancer cell death.
🔹 For Neuroprotection
In Alzheimer’s and Parkinson’s, neurons may die via ferroptosis due to iron accumulation and oxidative stress. ER-protective antioxidants could become a new class of neuroprotective drugs.
The McGill team’s probes also offer a powerful screening platform: researchers can now test whether experimental drugs truly block ferroptosis at its source—not just mask downstream effects.
Why This Matters to Clinical Microbiology
While ferroptosis is often studied in oncology and neuroscience, it also intersects with infectious disease and immunology:
- Some pathogens manipulate host cell death pathways to survive.
- Immune cells may use ferroptosis to eliminate infected cells.
- Chronic inflammation can promote lipid peroxidation, linking infection to tissue damage.
Understanding the subcellular geography of cell death helps microbiologists see how microbes interact with host cell biology—and how to tip the balance in favor of the patient.
Final Thought: Seeing Is Believing—and Healing
For decades, cell death was inferred from snapshots—fixed cells, broken membranes, late-stage markers. Now, thanks to smart molecular probes, we can watch biology in motion.
As Dr. Cosa puts it:
“Understanding how new forms of cell death work is essential to building better therapies. You can’t fix what you can’t see.”
With this new window into ferroptosis, we’re one step closer to precision control over life and death at the cellular level.
Stay Ahead in Cellular & Molecular Medicine
At ClinicalMicrobiology.org, we translate cutting-edge cell biology into insights that matter for clinicians, researchers, and health innovators.
👉 Subscribe today for expert coverage of emerging mechanisms like ferroptosis, pyroptosis, and beyond—explained clearly, without hype.
Note: This article is based on original research from McGill University (published in Nature Chemistry, October 2025). All scientific credit belongs to Dr. Gonzalo Cosa and colleagues. Content has been fully rewritten for clarity, originality, and educational value.





Post Comment